Singapore is often regarded as world-class in a number of subjects, but I’m not sure if many people realise in how many it is the world’s best. I get the sense that some still believe in greener pastures abroad. After all, Singaporeans themselves still leave to study in the UK, America, Australia, although they have some of the absolute top universities at home.
One of the areas the city-state is particularly strong at is nanotechnology.
Nanyang Technological University (NTU) regularly tops global rankings in the field, like the Shanghai one, where it has been an undisputed leader for the past several years. It also took the top spot in the US News rankings, based on metrics collected by Clarivate; and is also regularly in one of the top three spots in QS Material Sciences ranking.

Meanwhile, the National University of Singapore (NUS) is not far back, regularly landing in the top 10 of the same lists — for a tiny city-state competing with global superpowers like the USA or China, this is quite an amazing achievement.
But, for all its associations with engineering and material science, nanotechnology has its applications in life sciences and medicine as well. It’s also where the latest breakthrough, announced by NUS this month, happened.
It’s a product of its collaboration with Xiamen University in China: a cancer nanovaccine which not only completely clears solid tumours, but also induces a long-lasting immunity, preventing them from returning once removed.
In a breakthrough development, a team of scientists led by Narat Muzayyin Chair Professor Chen Xiaoyuan from the NUS Yong Loo Lin School of Medicine and Professor Liu Gang from Xiamen University has formulated a novel vaccine which showed high efficacy in the treatment of solid tumours, achieving complete clearance of solid tumours and inducing long-lasting immune memory.
– NUS press release
This prevents the relapse of tumour growth that the patient originally presented with and provides immunity against similar tumour types. This was proven through the application of this vaccine on melanoma tumour models.
Defeating more than just cancer
Even though effective and safe cancer therapies are the holy grail of medicine, it is worth noting that the technology used to make the vaccine can be used to treat other diseases as well.
Because its mode of action is focused on using the body’s own immune system to stimulate desirable response, it is more of a platform that can have a wide range of applications, rather than a solution to a single, specific health issue.
We are excited at this platform technology’s potential for further application in other diseases as well, such as chronic viral infection, in which T-cell exhaustion often occurs during infection and prevents the optimal viral control.
– Chen Xiaoyuan, Narat Muzayyin Chair Professor, NUS
When can it become available?
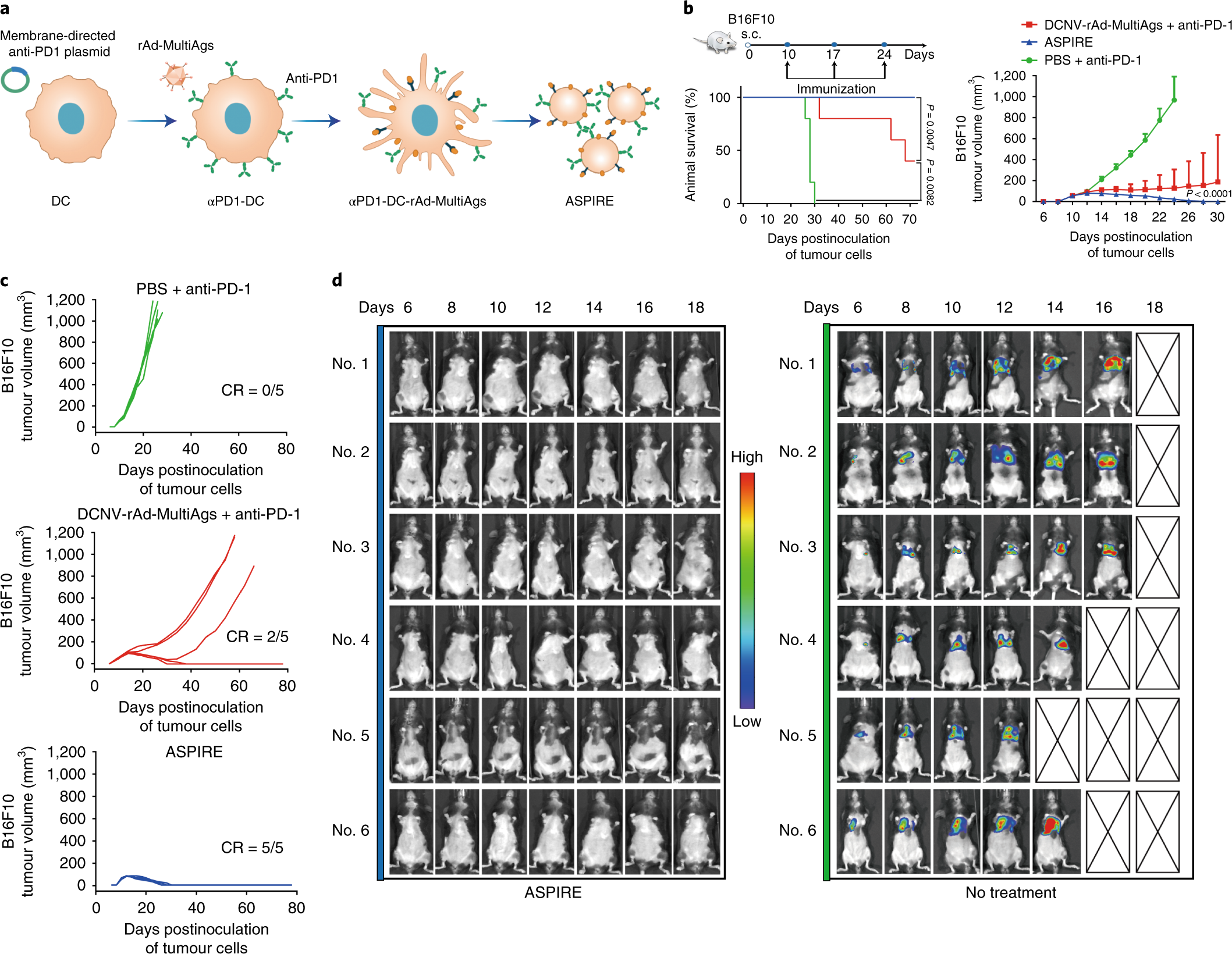
As usual with novel developments, the path from discovery to therapy can take a few years. So far, this method has proved to work excellently on mice, achieving 100 per cent cancer regression and long-lasting immunity to cancer regrowth.
The team is now going to work on standard operating procedures allowing a scaled synthesis of the vaccine, including proper quality control.
While there is no word on human trials, blood samples from human volunteers were used in the study. The overall outlook is optimistic as the technology used is not new in itself and can be adapted to larger scale deployment of the treatment, giving hope to cancer patients around the world that they may not only be spared the disease, but also invasive treatment.
…although the production of the ASPIRE formulation is somewhat more complicated than that of a DC vaccine, the chemistry, manufacturing and control of ASPIRE are not a major concern as we have established a standard operating procedure for a scaled synthesis with a proper quality control of the membrane vesicles.
– “A nanovaccine for antigen self-presentation and immunosuppression reversal as a personalised cancer immunotherapy strategy”, Nature Nanotechnology 2022

In conclusion, we have developed a novel nanovaccine platform with the ability to activate the immune response and break immune tolerance. We have demonstrated its ability to stimulate powerful CTL responses and enhance the immune checkpoint blockade with a remarkable therapeutic efficacy.
Owing to the tolerance of ASPIRE to a large protein insertion in a natural form, our approach provides a powerful and facile way to produce personalised cancer vaccines. Furthermore, this platform technology may be generally applicable to treat other diseases as well.
– “A nanovaccine for antigen self-presentation and immunosuppression reversal as a personalised cancer immunotherapy strategy”, Nature Nanotechnology 2022
The future is looking a bit brighter today.
Featured Image Credit: lightsource / depositphotos